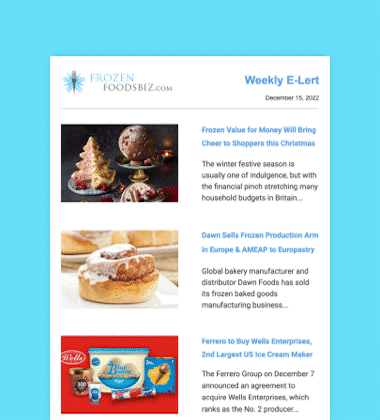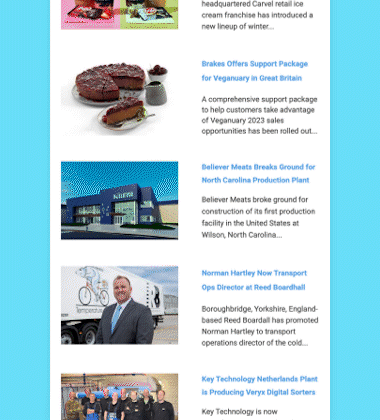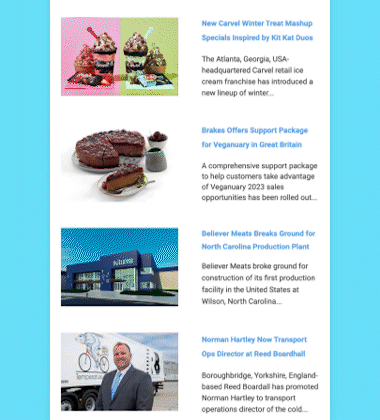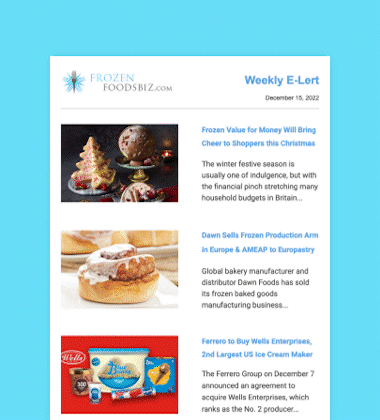
After an exhaustive scientific review that spanned two decades, the US Food and Drug Administration (FDA) determined on November 19 that AquAdvantage salmon is as safe to eat as any non-genetically engineered (GE) Atlantic salmon, and is also as nutritious.
FDA scientists rigorously evaluated extensive data submitted by the producer, AquaBounty Technologies, and other peer-reviewed documentation, to assess whether AquAdvantage salmon met the criteria for approval established by law – namely, safety and effectiveness. The data demonstrated that the inserted genes remained stable over several generations of fish, that food from the GE salmon is safe to consume by humans and animals, that the genetic engineering is safe for the fish, and the salmon meets the sponsor’s claim about faster growth.
The long awaited decision was applauded by Ronald L. Stotish, chief executive officer of Maynard, Massachusetts-headquartered AquaBounty. He called it a “game changer that brings healthy and nutritious food to consumers in an environmentally responsible manner without damaging the ocean and other marine habitats.”
The Center of Food Safety, a consumer advocacy group, has vowed to file a lawsuit challenging the FDA’s approval of the application.
AquAdvantage salmon is genetically altered to reach market size in less time than conventionally farmed salmon. It contains a growth hormone from Chinook salmon and a genetic switch from ocean pout that keeps the transplanted gene active non-stop, as opposed to part-time activity of the salmon’s own growth hormone. The bottom line is that fish grows to market size and can be harvested in 18 to 20 months, compared to 28 to 36 months for conventionally cultured salmon.
After assessing the environmental impacts of approving AquaBounty’s application, the FDA concluded that granting approval would not have a significant impact on the environment of the United States. That’s because the multiple containment measures the company will use in the land-based facilities in Panama and Canada make it extremely unlikely that the fish could escape and establish themselves in the wild.

At the moment the salmon is farmed exclusively in Panama, using eggs sourced from Prince Edward Island, Canada. Production capacity is approximately 100 tons per year.
Opponents of genetically engineered farming, among them elected officials from Alaska, have voiced concern that escaped fish could have a detrimental effect on the health of wild salmon. Alaskan waters are, by far, the nation’s primary source of wild-caught salmon.
“This harebrained decision goes to show that our federal agencies are incapable of using common sense,” said Congressman Don Young, a Republican.
William Muir, a professor of animal sciences at Purdue University, has a different point of view. “In contrast, the current practice of using wild-caught salmon as a food source is not sustainable,” he said. “Our oceans are overfished. This development provides a safe and sustainable alternative.”
A number of environmental and consumer groups have fiercely fought against the proposal on food safety grounds, effectively delaying the FDA’s decision for years. A public comment period, which began last September, generated 1.5 million statements of opposition against the GE salmon application. Furthermore, it has been reported that Obama White House personnel maneuvered behind the scenes to postpone approval for political reasons.
Meanwhile, Kroger and other retail supermarket chains in the United States are on record saying they will not sell GE salmon, even with FDA approval.
“This unfortunate, historic decision disregards the vast majority of consumers, many independent scientists, numerous members of Congress and salmon growers around the world who have voiced strong opposition,” declared Wenonah Hauter, executive director of Food & Water Watch.
GE proponents insist that growth hormone gene transplantation poses no safety or environmental risks. In a joint letter to President Barack Obama last year, a contingent of 90 scientists and biotechnology experts wrote: “Protests an anti-technology interests are, we believe the root cause of the unconscionable delays in FDA approval. These groups’ criticisms are based on misplaced economic/marketplace concerns and reactionary fears of people who either don’t understand or choose not to understand the science behind the AquAdvantage salmon.”
AquaBounty, a publicly traded company whose largest shareholder is Intrexon Corporation, says that the use of its technology by the land-based aquaculture industry will encourage more sustainable farming close to major consumer markets. Pointing out that currently the United States imports more than 95% of its Atlantic salmon supplies, weighing in at more than 200,000 tons per year, AquaBounty says that its technology offers “the opportunity for an economically viable domestic aquaculture industry” as well as reduced carbon footprint and “importantly, an alternative approach to fish farming that does not exploit the oceans.”
FDA Offers Food Labeling Guidance
The FDA’s decision not to require GE labeling for AquAdvantage salmon has been received negatively by those who believe it deprives shoppers of their ability to make a truly informed choice.
 “Consumers deserve to know what type of food they’re buying – and an overwhelming majority has told us that they want genetically modified food labeled in poll after poll,” said Michael Hansen, senior scientist with Consumers Union.
“Consumers deserve to know what type of food they’re buying – and an overwhelming majority has told us that they want genetically modified food labeled in poll after poll,” said Michael Hansen, senior scientist with Consumers Union.
“Although the law does not require food containing ingredients derived from these salmon to be labeled as GE, FDA recognizes that many consumers are interested in this information, and some food manufacturers will want to make the distinction,” the agency said in a statement.
As such, it is releasing two guidance documents detailing the FDA’s perspective on labeling – a draft guidance for labeling of food derived from Atlantic salmon that has or has not been genetically engineered, and a final guidance for labeling of food that has or has not been derived from GE plants – to help suppliers who wish to voluntarily make the distinction on the labeling of their food products.
“Both guidance documents explain FDA’s best thinking on how to make it easy for consumers to know whether a food was produced using genetic engineering or not,” said Felicia Billingslea, director of FDA’s Division of Food Labeling and Standards.