The Trader Joe’s grocery chain in the United States is recalling frozen Organic Acai Bowls. A notice at its website advises consumers who may have packages of the 10 oz (284g) product stored in home freezers to discard them, as they may contain plastic. The Bowls, which feature berries, bananas, granola and dried coconut, may be returned to the place of purchase to claim a refund.
“At Trader Joe’s, nothing is more important than the health and safety of our customers and crew members. With this in mind, we do the daily work to make certain our products meet our stringent food safety expectations. We don’t take any chances when it comes to product safety and quality,” declared a statement issued by the Monrovia, California-headquartered company.
Health Alert Issued for Meat and Poultry Pasties
Elsewhere on the North American frozen food front, the US Department of Agriculture’s Food Safety and Inspection Service (FSIS) has declared a public health alert for fully cooked ready-to-eat meat and poultry pasties due to misbranding and an undeclared allergen. The Pastry Oven brand products were made using an egg wash, a known allergen, that is not printed on the label. The alert has been issued to ensure that consumers with allergies to eggs are aware that this product should not be eaten.
Packed on or before last December 11, the following SKUs, all of which have best by dates of December 11, 2025 and prior, are subject to the public health notification:
• 8-oz. plastic packages containing The Pasty Oven Pasty with Chicken & Cheese.
• 8-oz. plastic packs containing The Pasty Oven Pasty Pizza with Pepperoni.
• 15-lb. cases containing 30 8-oz. units of The Pasty Oven Pasty with Chicken & Cheese.
• 15-lb. cases containing 30 8-oz. units of The Pasty Oven Pasty Pizza Pasty with Pepperoni.
The products, which bear establishment number “EST. 20650” inside the USDA mark of inspection, were sold at the establishment’s restaurant in Michigan and shipped to fundraiser groups including schools and non-profit organizations in Michigan, Minnesota and Wisconsin. They were not served at schools and are not part of the food provided by the USDA for the National School Lunch Program.
FSIS discovered the problem during routine labeling review activities when it found that the egg ingredient was not listed on the label. While there have been no confirmed reports of adverse reactions due to consumption of the pasties, there is concern that some products may be in home freezers.

Probe Ongoing in Frozen Supplemental Shakes and Listeria Cases
Meanwhile, the US Food & Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC), in collaboration with state and local partners, are investigating illnesses in a multistate outbreak of Listeria monocytogenes (Lm) infections linked to Lyons ReadyCare and Sysco Imperial Frozen Supplemental Shakes.
Late last November, the FDA was notified about an outbreak of Listeria monocytogenes (Lm) in the United States, with many ill people residing in long-term care facilities prior to illness onset. The agency’s traceback investigation identified that each of the facilities that supplied invoice information for review from 2024 to present received a frozen supplemental shake of either the Lyons ReadyCare or Sysco Imperial brand. As part of this probe, it collected environmental samples and found the outbreak strain of Listeria.
According to CDC, this outbreak includes cases dating back to 2018, with 20 cases across 2024 and 2025, and is currently ongoing. Epidemiologic evidence in previous investigations were unable to identify a source of the outbreak. As of February 21, 2025, a total of 38 people infected with the outbreak strain of Lm have been reported from 21 states. Of the people for whom information is available, 37 have been hospitalized. Eleven deaths have been reported, and 34 of those hospitalized (89%) reported living in long term care facilities or were admitted to hospitals prior to becoming sick. Records reviewed from facilities indicated nutritional shakes were available to residents.
FDA has been informed that certain Lyons ReadyCare and Sysco Imperial Frozen Supplemental Shakes are being voluntarily recalled. It is working with the recalling firms. The investigation is ongoing, and more information will be provided as it becomes available.

On February 21 Lyons Magnus LLC announced that the recall is underway for 4 oz. Lyons ReadyCare and Sysco Imperial Frozen Supplemental Shakes due to the potential for the products to be contaminated with Listeria monocytogenes. The Fresno, California foodservice and ingredients took the action in response to a recall of the products by the manufacturer, Prairie Farms Dairy, from its facility in Fort Wayne, Indiana.
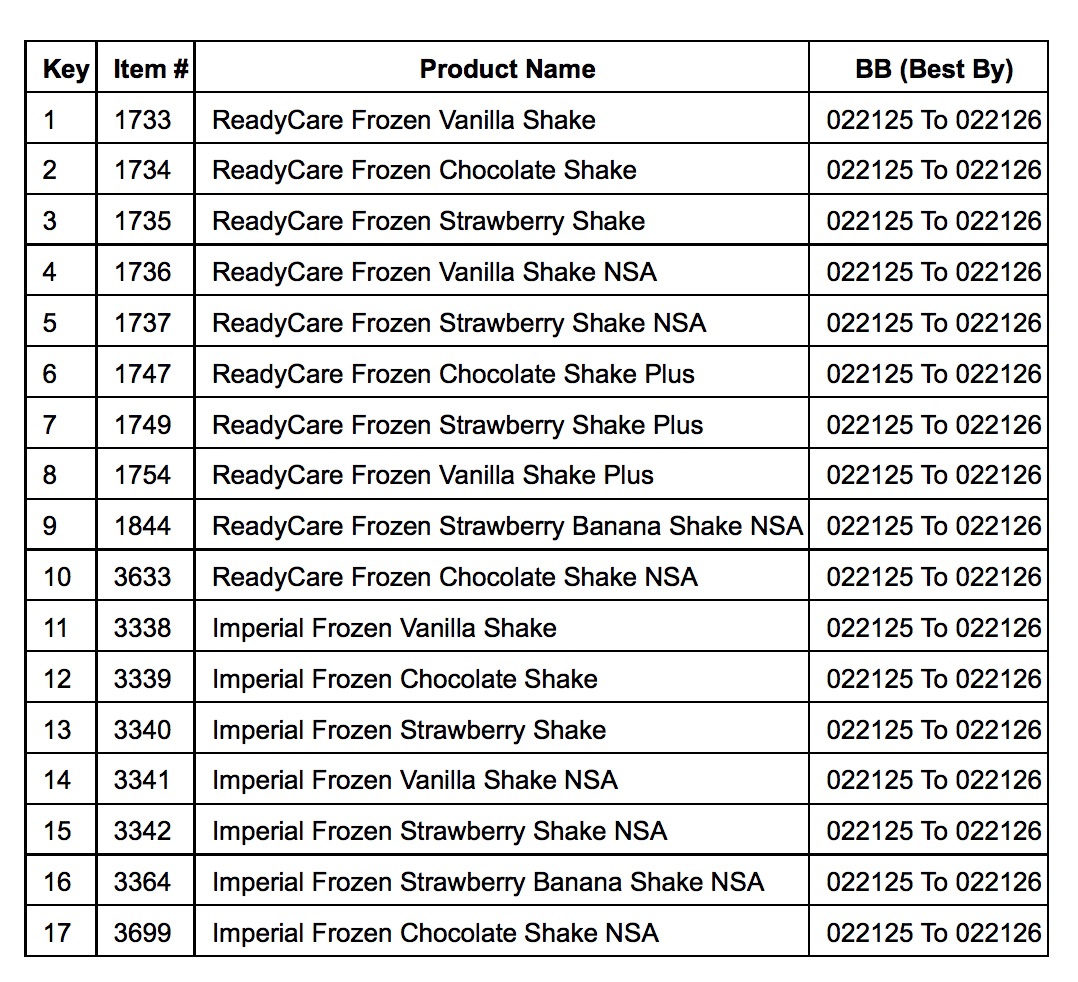
Lyons Magnus handled distribution of the recalled items, went primarily to long-term care facilities and were not available for retail sale. The frozen vanilla, chocolate and strawberry shakes were packed in 4 oz. cartons under the Lyons ReadyCare and Sysco Imperial brand names and shipped throughout the United States. The top of the carton has printing that identifies the Lot Code and Best By Date for these products.
A chart listing all recalled products is provided below: