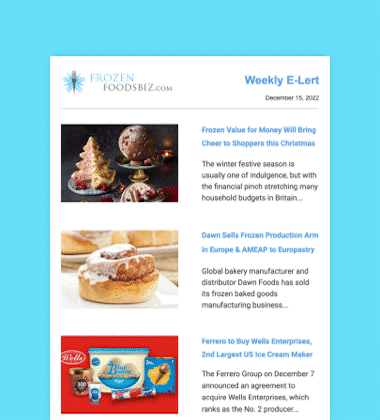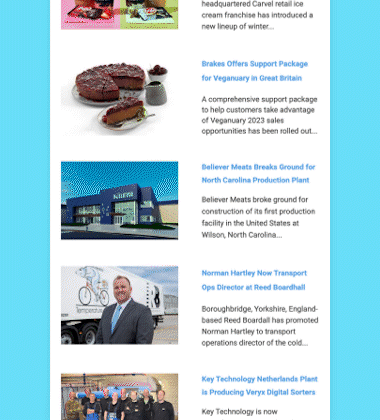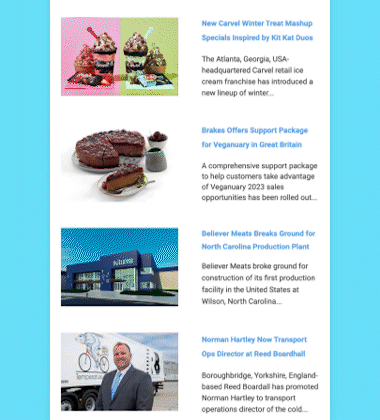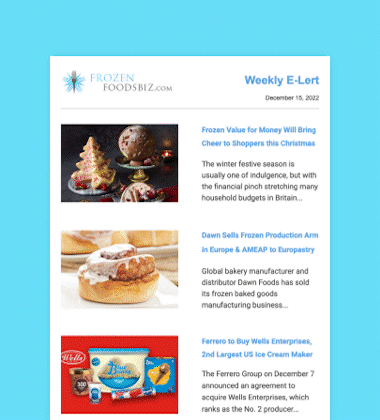
Maynard, Massachusetts-headquartered AquaBounty Technologies, Inc., a biotechnology company focused on enhancing aquaculture productivity through molecular genetics, has received approval from the US Food and Drug Administration (FDA) to raise AquAdvantage Salmon at its land-based contained facility near Albany, Indiana.
 A land-based recirculating aquaculture systems (RAS) in action.The product is a genetically engineered Atlantic salmon that can grow to market size in about half the time of traditional farmed Atlantic salmon, according to the company, a majority-owned subsidiary of Intrexon Corporation. The fish is farm-raised in indoor tanks featuring recirculating aquaculture systems (RAS).
A land-based recirculating aquaculture systems (RAS) in action.The product is a genetically engineered Atlantic salmon that can grow to market size in about half the time of traditional farmed Atlantic salmon, according to the company, a majority-owned subsidiary of Intrexon Corporation. The fish is farm-raised in indoor tanks featuring recirculating aquaculture systems (RAS).
The FDA initially gave the thumbs up to AquaBounty’s New Animal Drug Application (NADA) on November 19, 2015, authorizing production, sale, and consumption of AquAdvantage Salmon in the United States. As all such production facilities need separate site-specific approvals, the company submitted a supplementary NADA to the FDA requesting a green light to grow AquAdvantage Salmon at its farm site in Indiana. The facility as currently configured has production capacity of 1,200 tons per year and was designed to allow significant expansion. At this point, commercial production of AquAdvantage Salmon awaits only official labeling guidelines by the FDA.
 “This is another milestone in our journey to bring healthy and sustainable salmon to consumers. We are very pleased the FDA has continued their rigorous science-based review process and approved our application on its merits,” said AquaBounty CEO Ron Stotish. “Our Albany facility is within a few hours’ drive of major markets in Indianapolis, Chicago, Detroit, Cleveland, Columbus, Louisville and St. Louis, providing us with tremendous opportunity for growth. We still have work to do before we can start production, but we take great pride in this latest accomplishment.”
“This is another milestone in our journey to bring healthy and sustainable salmon to consumers. We are very pleased the FDA has continued their rigorous science-based review process and approved our application on its merits,” said AquaBounty CEO Ron Stotish. “Our Albany facility is within a few hours’ drive of major markets in Indianapolis, Chicago, Detroit, Cleveland, Columbus, Louisville and St. Louis, providing us with tremendous opportunity for growth. We still have work to do before we can start production, but we take great pride in this latest accomplishment.”