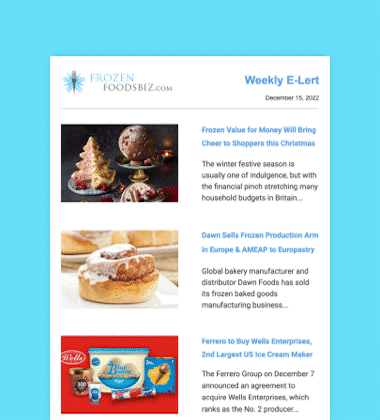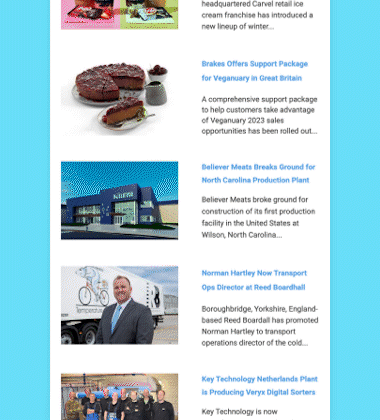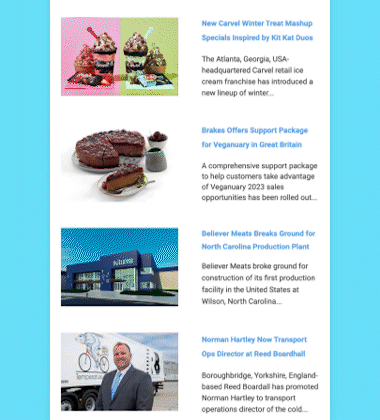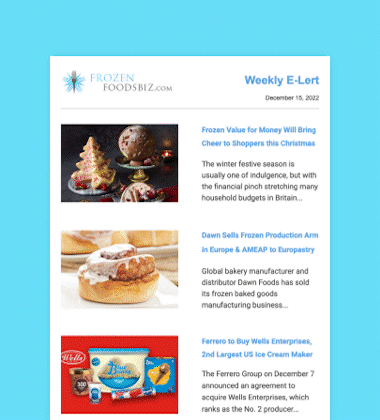
Seven Seas Fish Company, Ltd. (7 Seas) of Richmond, British Columbia, Canada, and John Heras, a 78-year-old owner who founded the company in 1967, were sentenced on December 6 in US District Court in Seattle to significant monetary fines and periods of probation for importation of previously refused food.
According to the US Attorney’s Office in the Western District of Washington, Heras admitted that between October of 2014 and August of 2015 Seven Seas (imported more than 9,000 pounds of potentially adulterated fish into the United States. The product had previously been refused entry because the US Food and Drug Administration (FDA) judged samples to be too decomposed and putrid.
At the sentencing hearing, Magistrate Judge Mary Alice Theiler remarked: “This activity leads consumers to be concerned about food safety.”
It should be pointed out that the FDA has not found any illness linked to those who consumed the fish.
US Attorney Brian T. Moran stated: “On two prior occasions, this company put its financial success over the food import regulations and the safety of consumers. Now, with a third strike, it is appropriate that the company and its part-owner face a federal criminal conviction and its consequences.”

According to records filed in the case, in June of 2014, Seven Seas purchased 12,100 pounds of frozen corvina, a whitefish similar to sea bass that is often served in ceviche, for $36,375 from a seafood company in Mexico. It initially attempted to have the shipment imported into the US at the Otay Mesa Port of Entry. However, when FDA consumer safety officers examined the fish, they determined that one-third of the product sampled was more than 20% spoiled and thus entry was refused. However, Seven Seas arranged for the shipment to be lawfully forwarded through the United States to its plant in Richmond, B.C., claiming that the product would be distributed in Canada.
After the corvina arrived in Canada, Heras cooked and ate some of the batch and claimed he found nothing wrong with it, according to the US Attorney’s Office. Despite his knowledge that the whitefish had been refused entry to the US, he reportedly encouraged others within the Seven Seas organization to sell the product to customers in the state of Washington and elsewhere. Some 9,020 pounds were imported into the United States without the required notice to the Secretary of Health and Human Services.
The company was ordered to pay a $150,000 fine within six months of the sentencing. Furthermore, for three years it will be on probation with increased scrutiny and surveillance of its imports. Heras, who no longer has an active leadership role in the business, will pay a $2,000 fine and will be on probation for one year.
Seven Seas has a “tarnished record regarding its compliance with import regulations,” according to a press release issued by the US Department of Justice. It stated, in part:
“In 2008, Canadian salmon owned by the company was seized because it was sold in violation of Canadian law and the Lacey Act. The fish, worth nearly $100,000, had been caught by illegal gill netting. Just one year later, in 2009, Seven Seas was fined $50,000 for selling salmon without notifying regulators after the fish had been detained because it was found unfit for human consumption. The fish was sold for mink feed, but without the required notice to the agency that had issued the detainer.:
The case was investigated by the FDA Office of Criminal Investigation, Customs and Border Protection (CBP), and Homeland Security Investigations. It was prosecuted by Assistant United States Attorney Matthew Diggs.

Statement from Seven Seas
On December 9, three days after the sentencing, Seven Seas Fish Co. CEO George Heras commented on the case in the following statement:
“We can assure you, Seven Seas takes the quality of our products and the safety of our customers very seriously – and we have done so for more than 50 years. We have vigorously defended the quality and safety of the 2014 fish shipment in question. The lesser misdemeanor plea reflects this.
“Various laboratory and expert analysis by third-parties, as well as by Seven Seas, concluded the fish shipment was of the quality we expect – and met food quality and safety standards. The FDA lab test found no abnormal bacteria counts. Our shipment was rejected through an organoleptic (sight and smell) analysis by FDA personnel who determined a portion of the samples didn’t meet a certain quality threshold.
“We understand that exporting products into the US is a privilege, not a right. While we acknowledge our role in this matter and understand how our approach and communications with authorities should have been handled more proactively, at no time did we put customer health and safety at risk or accept and distribute sub-par quality.
“Importantly, we have learned from the event in question and have invested in more modernized and regimented processes, tools and training to ensure rigorous regulatory compliance and quality management. Our company founder, John Heras, is no longer active in the business.
“We participate and comply with many inspection and quality programs undertaken by governments and customers alike. We are a HACCP/QMP and SQF Level II certified facility, as well as a CFIA and US FDA approved plant. We also embrace sustainability as a responsibility. We are a recognized partner of the Ocean Wise Conservation program, Marine Stewardship Council certified and a member of Sea Choice. We continue to meet and exceed all standards, ratings and audits. All of our entries to the U.S have cleared in 2019 (~ 99.9% since 2016).
“As part of this case, we disagree with the characterizations of two previous events in 2008 and 2009 that were cited. Neither were criminal nor impacted the quality of our products and the safety of our customers.
“You may have questions. We wish to be fully transparent. So, we’d like to provide you the same facts we gave as statement of record in court.
“The attorney stated that in 2008, ‘Canadian salmon owned by the company was seized because it was sold in violation of Canadian law” as a result of illegal gill netting.’ Seven Seas was not a direct party to this.
“We worked with a buying agent who sourced fish from the Fraser River, which has many suppliers from First Nations fishermen. The buying agent represented to Seven Seas that it had made some deals with First Nations fishermen, and that Canadian First Nations fishing entities had made arrangements to barter fish with other US First Nations. Some of the fish – meant for the buying agent and First Nations, and never bought or sold by Seven Seas – was unfortunately co-mingled with fish that had been lawfully caught and purchased by Seven Seas.
“All of the fish was then transported together for processing. Unfortunately, this resulted in an inability to track which fish had been legally caught and purchased by Seven Seas, and which fish were bartered among the “First Nations entities that were destined elsewhere. All of the fish was seized in lot. Seven Seas did not challenge the seizure as it (1) would have involved sorting the fish one by one to establish which were the fish legally purchased by Seven Seas and, (2) most importantly, we could not guarantee the traceability of the product. We no longer work with this buying agent.
“The attorney stated that in 2009, Seven Seas was fined $50,000 for selling salmon without notifying regulators after the fish had been detained because it was found unfit for human consumption. This is misleading. The fish in question was never intended for human consumption – it was from a roe fishery and the end-of-life-cycle salmon was to be ground and sold for pet food. It was cited that we processed the fish for pet food instead of having the fish “hold intact” (“as is”), which in our judgment likely would lead to spoiling, during their review period. This statement is correct. After the original sample at the border failed inspection, Seven Seas worked with the Food and Drug Administration and U.S. Customs to verify the traceability of the pet food and roe/caviar products. The products were then shipped to Vancouver.
“In both instances, we fully co-operated with the FDA and U.S. Customs. In both instances, the quality of our products and the safety of our customers were not affected.
“We are dedicated to continuous quality improvement. We have learned from this event and continue to invest in training, procedures and technology to ensure we comply with all shipping regulations and authorities around the world. We stand by our people and the quality of our products, and you can trust in the shipments provided by Seven Seas.”